Abstract
We have studied the long-term toxicity of a Roundup-tolerant GM maize (NK603) and
a whole Roundup pesticide formulation at environmentally relevant levels from 0.1 ppb.
Our study was first published in Food and Chemical Toxicology (FCT) on 19 September, 2012. The first wave of criticisms arrived within a week,
mostly from plant biologists without experience in toxicology. We answered all these
criticisms. The debate then encompassed scientific arguments and a wave of ad hominem and potentially libellous comments appeared in different journals by authors having
serious yet undisclosed conflicts of interests. At the same time, FCT acquired as
its new assistant editor for biotechnology a former employee of Monsanto after he
sent a letter to FCT to complain about our study. This is in particular why FCT asked
for a post-hoc analysis of our raw data. On 19 November, 2013, the editor-in-chief requested the
retraction of our study while recognizing that the data were not incorrect and that
there was no misconduct and no fraud or intentional misinterpretation in our complete
raw data - an unusual or even unprecedented action in scientific publishing. The editor
argued that no conclusions could be drawn because we studied 10 rats per group over
2 years, because they were Sprague Dawley rats, and because the data were inconclusive
on cancer. Yet this was known at the time of submission of our study. Our study was
however never attended to be a carcinogenicity study. We never used the word ‘cancer’
in our paper. The present opinion is a summary of the debate resulting in this retraction,
as it is a historic example of conflicts of interest in the scientific assessments
of products commercialized worldwide. We also show that the decision to retract cannot
be rationalized on any discernible scientific or ethical grounds. Censorship of research
into health risks undermines the value and the credibility of science; thus, we republish
our paper.
Keywords:
Conflicts of interests; Confidentiality; Retraction; GMO; Roundup; Glyphosate; NK603 There is an ongoing debate on the potential health risks of the consumption of genetically We first published these results in Food and Chemical Toxicology (FCT) on 19 September, 2012 [3] after a careful and thorough peer review. However, 1 year and 2 months later, in An initial study on NK603 maize was submitted by Monsanto Company in support of commercial We found that these products provoked statistically discriminant disturbances in biochemical These findings were immediately dismissed by persons involved in the products’ authorizations, Overall, the first wave of criticisms arrived within a week, mostly from plant biologists. Paul Christou, the lead author of Arjo et al. [13], demanded that our paper be retracted and insulted us personally. He claimed first Christou is not only the editor-in-chief of Transgenic Research, the journal in which The Arjo et al. article was co-authored by Wayne Parrott, an active member of the In addition, Christou and his co-authors made numerous mistakes, false and unsubstantiated Arjo et al.’s paper begins with a false assertion that is not evidenced in the paper Importantly, we had already answered many of the criticisms of our paper made by Arjo Christou was not alone in failing to declare conflicts of interest in his criticism All of this has huge implications for public health. We observed an intense lobbying In February 2013, FCT acquired a new assistant editor for biotechnology, Richard E. In fact, we can question the independence of this re-evaluation. After his appointment We received a letter from the editor-in-chief of FCT, A. Wallace Hayes, asking us Following the retraction of our paper, many letters were sent to the editor-in-chief According to an editorial in Environmental Health Perspectives [46], ‘the decision to retract a published scientific work by an editor, against the desires Recent reviews of the GM food safety literature have found that research concluding Transparency of, and access to, all the raw data obtained by companies and accepted The second step must be the building of new experiments for new or the most important The reason given to retract our paper - ‘inconclusiveness’ - is unprecedented and violates the norms of scientific publishing. The decision The authors declare that they have no competing interests. GES designed and coordinated the commentary. RM participated in the drafting of the We acknowledge the Charles Leopold Mayer (FPH) and Denis Guichard Foundations, together Seralini G-E, Mesnage R, Clair E, Gress S, de Vendomois J, Cellier D (2011) Genetically modified crops safety assessments: present limits and possible improvements. Environ Sci Eur 23:10 BioMed Central Full Text
Spiroux de Vendômois J, Cellier D, Velot C, Clair E, Mesnage R, Seralini GE (2010) Debate on GMOs health risks after statistical findings in regulatory tests. Int J Biol Sci 6:590-598 Publisher Full Text
Seralini GE, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendomois JS (2012) RETRACTED: Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically Retracted in Food and Chemical Toxicology. 2014, 4263: 4244
Hammond B, Dudek R, Lemen J, Nemeth M (2004) Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant
Spiroux de Vendômois J, Roullier F, Cellier D, Seralini GE (2009) A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci 5:706-726 Publisher Full Text
(2012) Review of the Séralini et al. (2012) publication. EFSA J 10(10):2910
[http://www.efsa.europa.eu/fr/press/news/130114.htm] webcite EFSA: EFSA Promotes Public Access to Data in Transparency Initiative (2013). Press Release .
Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini GE (2005) Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ Health Perspect 113:716-720 Publisher Full Text
Benachour N, Seralini GE (2009) Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic,
Mesnage R, Bernay B, Seralini GE (2013) Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human
Hammond B, Goldstein DA, Saltmiras D (2013) Letter to the editor. Food Chem Toxicol 53:459-464 Publisher Full Text
Seralini GE, Mesnage R, Defarge N, Gress S, Hennequin D, Clair E, Malatesta M, de Vendomois JS (2013) Answers to critics: why there is a long term toxicity due to NK603 Roundup-tolerant
Arjo G, Portero M, Pinol C, Vinas J, Matias-Guiu X, Capell T, Bartholomaeus A, Parrott W, Christou P (2013) Plurality of opinion, scientific discourse and pseudoscience: an in depth analysis
Houllier F (2012) Biotechnology: bring more rigour to GM research. Nature 491:327 Publisher Full Text
Martinelli L, Karbarz M, Siipi H (2013) Science, safety, and trust: the case of transgenic food. Croat Med J 54:91-96 Publisher Full Text
Romeis J, McLean MA, Shelton AM (2013) When bad science makes good headlines: Bt maize and regulatory bans. Nat Biotechnol 31:386-387 Publisher Full Text
King-Herbert A, Sills R, Bucher J (2010) Commentary: update on animal models for NTP studies. Toxicol Pathol 38:180-181 Publisher Full Text
[http://www.google.com/patents/US5015580] webcite US Patent 5015580: Particle-mediated transformation of soybean plants and lines. 1991, .
[http://www.google.com/patents/US5554798] webcite US Patent 5554798: Fertile glyphosate-resistant transgenic corn plants. 1996, .
[http://www.jic.ac.uk/corporate/media-and-public/news-archive/010716.htm] webcite John Innes Centre: Laying the foundation for more science at the John Innes Centre, Norwich, UK. 2001, .
[http://publicationethics.org/files/retraction%20guidelines.pdf] webcite COPE: Retraction guidelines. 2009, .
(2013) Biotechnology Update Symposium.
[http://www.nrdc.org/media/pressreleases/060131.asp] webcite NRDC: Industry association barred from influencing international health standards. 2006, .
Robinson C, Holland N, Leloup D, Muilerman H (2013) Conflicts of interest at the European Food Safety Authority erode public confidence. J Epidemiol Community Health 67(9):712-720
Lougheed T (2006) WHO/ILSI affiliation sustained. Environ Health Perspect 114(9):A521 Publisher Full Text
[http://www.google.co.in/patents/US7005561] webcite US Patent 7005561: Arabitol or ribitol as positive selectable markers. 2006, .
[http://www.google.com/patents/US6096523] webcite US Patent 6096523: Transformation vector system. 2000, .
Hayes AW (2014) Editor in Chief of Food and Chemical Toxicology answers questions on retraction. Food Chem Toxicol 65:394-395 Publisher Full Text
Worstall T: Proof perfect that the Seralini paper on GM corn and cancer in rats is rubbish..
Sourice B: OGM: la guerre secrète pour décrédibiliser l’étude Séralini. 2012, .
Matthews J: Smelling a corporate rat. 2012, .
Commission E: 16/10/2012 Press Statement. Brussels: ?; 2012. MEMO/12/788.
(2012) EFSA Management Board Chair Resigns.
[http://www.hesiglobal.org/i4a/pages/index.cfm?pageid=3595] webcite ILSI: Symposium on sensitizing properties of protein. 2012, .
(2005) ILSI Protein Allergenicity Technical Committee.
Mezzomo BP, Miranda-Vilela AL, de Souza Freire I, Barbosa LC, Portilho FA, Lacava Mezzomo B, Miranda-Vilela A, Freire I, Barbosa L, Portilho F (2013) Hematotoxicity of Bacillus thuringiensis as spore-crystal strains Cry1Aa, Cry1Ab, doi:104172/jhtd1000104
Hayes AW (2014) Retraction notice to “Long term toxicity of a Roundup herbicide and a Roundup-tolerant
Meyer H, Hilbeck A (2013) Rat feeding studies with genetically modified maize - a comparative evaluation of
(2012) OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects Test No. 452:
Séralini GE, de Vendomois JS, Cellier D, Sultan C, Buiatti M, Gallagher L, Antoniou M, Dronamraju KR (2009) How subchronic and chronic health effects can be neglected for GMOs, pesticides or
Doull J, Gaylor D, Greim HA, Lovell DP, Lynch B, Munro IC (2007) Report of an Expert Panel on the reanalysis by of a 90-day study conducted by Monsanto
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose
Portier C, Goldman L, Goldstein B (2014) Inconclusive findings: now you see them, now you don’t! Environ Health Perspect 122(2):A36
doi:10.1289/ehp.1408106
Diels J, Cunha M, Manaia C, Sabugosa-Madeira B, Silva M (2011) Association of financial or professional conflict of interest to research outcomes
[http://www.criigen.org/SiteFr//images//anses_letter.pdf] webcite Mortureux M: ANSES letter regarding the raw data of glyphosate. 2013,.
Mesnage R, Defarge N, SpirouxDeVendômois J, Séralini GE (2014) Major pesticides are more toxic to human cells than their declared active principles. BioMed Res Int 2014:Article ID 179691 Publisher Full Text
German Federal Agency BfR: The BfR has finalised its draft report for the re-evaluation of glyphosate..
Nielsen KM (2013) Biosafety data as confidential business information. PLoS Biol 11(3):e1001499 Publisher Full Text
Background
modified (GM) plants containing high levels of pesticide residues [1]. Currently, no regulatory authority requests mandatory chronic animal feeding studies
to be performed for edible GMOs and formulated pesticides. This fact is at the origin
of most of the controversies. Only studies consisting of 90-day rat feeding trials
have been conducted by manufacturers for GMOs. Statistical differences in the biochemistry
of treated rats versus controls may represent the initial signs of long-term pathologies
[2], possibly explained at least in part by pesticide residues in the GM feed. This is
why we studied the long-term toxicity of a Roundup-tolerant GM maize (NK603) and a
whole Roundup pesticide formulation at environmentally relevant levels from 0.1 ppb.
an unusual step, the editor-in-chief requested the retraction of our study, while
conceding that the data were not incorrect and that there was no misconduct and no
fraud or intentional misinterpretation. According to him, some data were inconclusive,
but for reasons already known at the time of submission of the paper. The present
paper is a summary of the debate resulting in this retraction, which in our view is
a historic example of conflicts of interests in the scientific assessments of products
commercialized worldwide.
The long-term toxicity study of the NK603 maize and Roundup
authorization of the maize. NK603 maize was fed to 4 groups of 20 Sprague Dawley rats
(2 doses of 11% and 33% in the diet of both sexes) for 90 days [4]. The blood analyses were performed on 10 rats per group. The re-analysis of the raw
data resulted in a debate on the biological relevance of admitted statistical differences
versus controls as the first signs of hepatorenal toxicities [5]. To solve the problem, a 2-year-long study was carried out using two hundred Sprague
Dawley rats to which the following treatments were administered: NK603 maize treated
or not with Roundup at three different levels in their feed (11%, 22%, and 33% of
the total diet) and Roundup alone, administered via drinking water at three different concentrations, from the admitted residual level
in regular tap water (0.1 ppb), to the maximum level authorized in GMOs (400 ppm),
up to half of the agricultural dose (0.5%). They were divided into ten groups, each
containing ten males and ten females. No other long-term study has examined the effects
of regular consumption of Roundup-tolerant GM maize and of a pesticide formulation,
in any dilution, on blood parameters, sexual hormones, and multiple organs.
markers of livers and kidneys in females at the 15th month, when most of the rats
were still alive. At the same time, testosterone and estradiol levels were also disturbed.
At the end of the experiments, these disrupted biochemical markers corresponded to
pathologies evidenced in a blinded manner: notably hepatorenal deficiencies, more
severe in males, and female mammary tumors, which led to premature deaths. For instance,
after around 700 days, there were up to 3.25 more mammary tumors (the highest rate
was observed in females consuming 0.1 ppb of Roundup in water). This could be associated
with a 2.4-time increase in pituitary dysfunctions noticed by the end of the experiment
(2 years).
or in collaboration with biotech industries. A number of them wrote to FCT to nourish
a controversy, including Richard Goodman, a former Monsanto employee in charge of
the immunotoxicity files of GMOs, and Paul Christou, a patent holder of the methods
used to create transgenic plants. This was rapidly followed by a coordination of national
regulatory agencies organized by the European Food Safety Authority (EFSA), released
on 4 October, 2012 [6]. The EFSA had previously assessed NK603, and glyphosate, the declared active principle
of Roundup, as safe on the basis of regulatory data, which they never fully published.
The EFSA has since published Monsanto’s safety data on NK603 maize [7], but not on glyphosate. The NK603 data are in a pdf format preventing an easy statistical
re-analysis. However, there was no long-term toxicological assessment for NK603, or
for Roundup. Moreover, we demonstrated in several studies [8-10] that Roundup is far more toxic than glyphosate because of non-inert adjuvants. On
10 October, 2012, the Monsanto Company also sent its criticisms to FCT [11] but did not release its safety data, claiming commercial confidentiality.
We answered all criticisms [12] in FCT on 9 November, 2012. The debate then encompassed scientific arguments. A second
wave of ad hominem and potentially libelous comments appeared in different journals [13-16]. Regrettably, there were no invitations to respond to these exacerbated attacks,
which we discovered only by our literature survey. Some of the authors of these articles
had serious yet undisclosed conflicts of interest. The scientific remarks concentrated
on the supposedly inadequate choice of the Sprague Dawley rat strain, which is, however,
a classic model for toxicology [17]. The Sprague Dawley strain was also used by Monsanto in its 90-day test on the same
maize [4]. In addition, Monsanto measured biochemically the same number of rats per group as
in our experiment. Thus, with regard to blood and urine biochemistry, Monsanto gathered
data from the same number of rats that we did.
Unsubstantiated allegations of fraud or errors
in a letter addressed to the editor-in-chief that the publication of our study ‘does not meet minimal acceptable standards of scientific rigor’ and ‘will damage an entire scientific discipline due to flawed conclusion’ (personal communication). Then, he attacked us in an article published in the journal
Transgenic Research on 20 December 2012 [13]. The quantity of insults and defamations in this paper, authorized and co-authored
by the editor-in-chief in a supposedly serious journal, is excessive. They include:
‘abject failure to treat the experimental animals in a humane manner’, ‘inability to formulate a valid hypothesis’, ‘media fanfare’, ‘fraudulent or knowingly inaccurate statements’, ‘unethical behavior’, ‘transparent attempt to discredit regulatory agencies’, ‘ammunition for extremists’, ‘flawed science’, ‘disingenuous or inept’, and ‘unjustified waste of animals’ (while at the same time asking for more animals in the groups). Christou and co-authors
suggest that by practising ‘flawed science’, we are working against ‘progress towards a better quality of life’ and in fact are ‘actively working to make life worse’. We were not invited to reply. This behaviour can be explained, though not justified,
by the undisclosed conflicts of interests.
he published his article, but is also linked to Monsanto [18]. He is named as the inventor on several patents on GM crop technology, for most of
which Monsanto owns the property rights. These include patents on the plant transformation
process [19] used to make glyphosate-tolerant transgenic corn plants [20]. He worked as a researcher at Agracetus Inc. (later acquired by Monsanto) for 12 years.
Then, from 1994 to 2001, Christou worked at the John Innes Centre in the UK [18], which is heavily invested in GM crop technology [21]. He thus has no mammalian toxicology background. However, in his published article,
Christou only gave as his affiliation his publicly funded position at a research institute.
Christou’s failure to declare his current interests - his inventor status on patents
concerning the company that developed the products we tested - could be considered
grounds for retraction of a paper in a scientific journal, according to ethical guidelines
for scientific publishing [22].
Biotechnology Committee at the International Life Sciences Institute (ILSI) [23]. ILSI is funded by multinational food, agribusiness, and biotechnology companies,
including Monsanto and Syngenta [24]. ILSI has proved highly controversial in North America and Europe due to its influence
on risk assessment methodologies for chemicals, pesticides, and GM foods [25-27]. Wayne Parrott also has an inventor status in patents on materials and methods for
selecting transgenic organisms [28] and transformation vector systems [29].
assertions, and misrepresentations of our data. The title of Arjo et al.’s paper includes
defamation and a misrepresentation of our research, implying that it is ‘pseudoscience’ and alleging that it claimed Roundup Ready maize and Roundup herbicide caused ‘cancer’ in rats - a claim we never made. We did not even use the word ‘cancer’ in our paper although this argument was reiterated in the final letter of the editor-in-chief
of FCT when explaining his decision to retract our paper [30]. Tumors do not always lead to cancer, even if they can be more deleterious in a shorter
time because of their size or body position, by hurting internal functions.
or in the cited source: ‘It started with a press conference in which journalists agreed not to engage in fact-checking’. The authors made other false assertions about our study, for example, alleging
that ‘the water consumption was not measured’. In fact, we measured both the water and food consumption, and the stability of
the Roundup solution over time. This was indicated in the paper, in which we explained
that all the data cannot be shown in one paper and that we concentrated on the most
important data; these parameters were only part of a routine survey. They also falsified
the reporting of the data, compiling the mortality data only at the end of the experiment
and ignoring the originality and the major findings of the differential chronological
effects between treated rats and controls, which we established by measuring tumor
size twice a week over 2 years. Moreover, we respected legal requirements and ethical
norms relating to animal experiments, and Arjo et al. present no evidence of the contrary,
so their allegation of inhumane treatment of the rats is without substance.
et al. in a paper that was published before that of Arjo et al. [12]. Their publication was received on 20 December 2012, when our paper was published
on 9 November 2012. Our published answers were simply ignored.
of our paper. Since we underlined that 75% of the comments addressed to FCT within
a week after our study was published came from plant biologists, it was discovered
that several had developed patents on GMOs. Some authors were employees of Monsanto
Company, which owns NK603 GM maize and sells Roundup herbicide [4,11]. Other more recent papers, published by plant biologists and/or affiliates of the
industry-funded group ILSI [15,16], repeated the arguments. The author of a separate article criticizing our study expressed
concern that our results could damage public opinion about GM crops [14] - a sentiment that gives precedence to economic interests over public health. An
article in Forbes magazine even alleged, without presenting any evidence, that we
had committed fraud [31]. Surprisingly, even Monsanto authors [11] declared that they had ‘no conflicts of interest’ in their first draft published
online on FCT website. Investigative reports [32,33] evidenced that many authors of these opinions had failed to disclose their conflicts
of interest, including Henry Miller, Mark Tester, Chris Leaver, Bruce Chassy, Martina
Newell-McGloughlin, Andrew Cockburn, L. Val Giddings, Sivramiah Shantharam, Lucia
de Souza, Erio Barale-Thomas, and Marc Fellous. The undisclosed conflicts of interest
included links with biotechnology companies that develop GMOs and with industry-backed
lobbying organizations.
in parliaments, as well as proofs of conflicts of interests for persons involved in
the regulatory decisions for the commercialization of these products [26]. A series of high-profile conflict-of-interest revelations (not restricted to GMOs
and pesticides) led to the resignations of leading administrators involved in decisions
affecting the assessment of these products, including the European Commissioner John
Dalli [34] and the former chair of the European Food Safety Authority’s (EFSA) management board
Diana Banati [35]. In February of 2013, a strange occurrence following the publication of our paper
raised questions about the connections of industry to scientific publishing, described
below.
Conflicts of interests in the editorial board
Goodman. The editor-in-chief has admitted that Goodman was introduced into the editorial
board after he sent a letter to FCT to complain about our study. In his letter, Goodman
appears worried about economic consequences but not so much about potential public
health consequences (personal communication). He wrote: ‘The implications and the impacts of this uncontrolled study is having HUGE impacts,
in international trade, in consumer confidence in all aspects of food safety, and
certainly in US state referendums on labelling’. Further in his letter, Goodman asked for ‘an evaluation by an independent set of toxicologists’. This is particularly why the Publishing Assistant for FCT asked for our raw data
on 15 March 2013.
at FCT, Goodman was a member of the subcommittee that requested our raw data, until
we complained to Elsevier publishing group. Goodman is far from being independent.
He previously worked for Monsanto for 7 years [36]. He also has a long-standing affiliation with ILSI [37]. Goodman will now deal with all biotechnology papers submitted to FCT. Another scientific
paper on GMO risks was withdrawn from FCT, without explanation shortly after it had
been accepted and published by the journal [38]. The paper was immediately published by another journal [39] according to the authors’ initiative.
to retract our paper on 19 November 2013, more than 1 year after its publication [40]. In his retraction notice, the editor-in-chief certifies that ‘no evidence of fraud or intentional misrepresentation of the data’ was found in the investigation, that the results are ‘not incorrect’, ‘there was no misconduct’, and that the sole reason for retraction is the ‘inconclusiveness’ of the paper. He argued that no conclusions could be drawn because we studied 10
rats per group over 2 years, because they were Sprague Dawley rats, and because we
could not conclude on cancer. In fact, the Sprague Dawley is a standard choice for
2-year studies performed by industry and independent scientists alike [17,41]. We also measured 10 animals per sex per group according to OECD 452 guideline on
chronic toxicity studies [42] because our study is a chronic toxicity study that was never intended to be a carcinogenicity
study. We wish to point out that Dr Hayes’ decision is in violation of the retraction
guidelines of the Committee on Publication Ethics (COPE), of which FCT is a member.
‘Inconclusiveness’ is not a valid reason for a journal to retract a paper. Lack of conclusiveness (which
can be discussed) and error are not synonymous. COPE criteria for retraction included
scientific misconduct/honest error, prior publication, plagiarism, or unethical research.
None of these criteria applied to our study. On the contrary, numerous published scientific
papers contain inconclusive findings. It is for further studies to build on the reported
findings and arrive at a more conclusive position. In contrast with our study measuring
toxicity, the Monsanto study reporting safety with the same number and the same strain
of rats, but limited to 90 days, [4] is not subject to the same controversy. The data in the Monsanto study show statistically
significant differences in multiple-organ functions between the GM and non-GM feeding
groups, which the authors dismissed as not ‘biologically meaningful’, using a set
of questionable criteria [43]. The significant effects observed do not have to be linear to the dose to be taken
into consideration; otherwise, endocrine effects will be dismissed. In addition, biochemical
disturbances do not have to correlate simultaneously with organ lesions, in contrast
to the claims of Doull et al. [44] in defence of Monsanto. These outdated concepts coming from the toxicology of poisons,
and are not valid for endocrine disruption [43,45]. If 10 rats/sex/group are too few to demonstrate a toxic effect, then this number
of rats is certainly too small to demonstrate safety. Overall, in the current system
of assessment, any toxic effect is first suspected to be a false positive, arising
by chance, rather than questioning whether no evidence of effect is a false negative
result. The Monsanto data as presented are thus inconclusive and should also be retracted.
of FCT. On 10 December 2013, he published a defence of the retraction, which raised
many doubts as to his understanding of our data [30]. He claimed that we concluded on cancer, although ours was a long-term toxicity study
with a detailed statistical analysis of blood and urine parameters. He also defended
the study done by Monsanto [4] claiming that they used 20 rats/sex/group while we only used 10 rats/sex/group. In
fact, despite the fact that the Monsanto study used twice our sample size, the Monsanto
authors only analyzed blood and urine from half of the animals (10), the same number
of sampled animals as in our study.
of the authors, because it is ‘inconclusive’ based on a post hoc analysis represents
a dangerous erosion of the underpinnings of the peer-review process, and Elsevier
should carefully reconsider this decision’.
Confidentiality and censorship erode the value of science
that GM products were safe tended to come from industry and that research conducted
by those with either financial or professional conflicts of interest was associated
with outcomes favorable to the GM sector [47]. In fact, it appears in our case that consequences of conflicts of interests in science
go beyond divergence in scientific interpretations and also rely on unscientific practices:
confidentiality and censorship.
by regulatory agencies (overall blood analyses of rats) as proof of safety for products,
is an unavoidable first step to move forward in this debate. It is the only way in
which the scientific community can enter the scientific discussion. This is why we
republish our paper in an open access way, together with its raw data allowing debate
about our results. This is not possible for the data used as a proof of safety for
commercial authorizations. The Monsanto toxicological data on NK603 maize recently
made public by EFSA is not in a statistically usable format and an agreement with
Monsanto is requested before use. Moreover, the data examined for Roundup authorizations
are clearly inadequate [48]. For instance, ANSES (French Agency for Food, Environmental and Occupational Health
& Safety), confirmed to us in writing (January 2013) that there were no 2-year studies
of Roundup in its whole formulation on animals, adding that there are a few studies
of acute toxicity (a few days up to 3 weeks) without any blood tests. Instead, glyphosate,
which is much less toxic than Roundup [10,49], is tested alone by Monsanto, in its reports to regulatory authorities [50]. We strongly emphasize that data with implications for public health are not related
to manufacturing patents and should not be kept confidential. Removal of confidentiality
claims on biosafety data is necessary to adhere to standard scientific procedures
of quality assurance, to increase transparency, to minimize impacts of conflicts of
interests, and ultimately to improve public confidence in GMOs [51]. Moreover, in the regulatory assessment of GMOs, chemicals, and medicines, confidential
tests are conducted by the applicant companies themselves, often in their own laboratories
or in those of subcontractors.
products, by laboratories independent of the companies. They will be recruited by
public tender, with compulsory transparency of the results. This public research will
be funded by companies, at a level corresponding to their previous budget for regulatory
testing, but managed independently of the companies. The protocols and results will
be submitted to open and contradictory assessments. Thus, there will be no additional
financial cost or time delay to the current system. Such reforms will not only radically
transform the understanding and knowledge of toxicology and science in general, but
will radically reduce public health costs and promote trust in companies and science.
This will move the world towards a sustainable development of products with low, if
any, impacts on health and environment.
to retract cannot be rationalized on any discernible scientific grounds. Censorship
on research into the risks of a technology so critically entwined with global food
safety undermines the value and the credibility of science.
Competing interests
Authors’ contributions
manuscript and final version. ND and JsDV helped in the writing, compiling the literature,
revising details, and proofreading the manuscript. All authors read and approved the
final manuscript.
Acknowledgements
with CRIIGEN, for fellowships and structural supports. We are equally thankful to
Malongo, Lea Nature, and the JMG Foundation for their help.
References
modified maize. Food Chem Toxicol 50:4221-4231
corn. Food Chem Toxicol 42:1003-1014 Publisher Full Text
and placental cells. Chem Res Toxicol 22:97-105 Publisher Full Text
cell toxicity. Toxicology 313:122-128 Publisher Full Text
genetically modified maize and to a Roundup herbicide. Food Chem Toxicol 53:461-468
of the Seralini et al. study claiming that Roundup Ready corn or the herbicide Roundup
cause cancer in rats. Transgenic Res 22:255-267 Publisher Full Text
[http:/ / www.forbes.com/ sites/ timworstall/ 2012/ 09/ 21/ proof-perfect-that-the-seralini-pap er-on-gm-corn-and-cancer-in-rats-is -rubbish/ ] webcite
[http:/ / blogs.rue89.nouvelobs.com/ de-interet-conflit/ 2012/ 11/ 12/ ogm-la-guerre-secrete-pour-decredib iliser-letude-seralini-228894] webcite
[http:/ / www.spinwatch.org/ index.php/ issues/ science/ item/ 164-smelling-a-corporate-rat] webcite
ZG, Grisolia CK: WITHDRAWN: Effects of oral administration of Bacillus thuringiensis
as spore-crystal strains Cry1Aa, Cry1Ab, Cry1Ac or Cry2Aa on hematologic and genotoxic
endpoints of Swiss albino mice.Food Chem Toxicol 2012, doi:10.1016/j.fct.2012.10.032.
Cry1Ac or Cry2Aa in Swiss albino mice. J Hematol Thromb Dis 1:104
genetically modified maize” [Food Chem. Toxicol. 50 (2012) 4221–4231]. Food Chem Toxicol 63:244 Publisher Full Text
applied methods and risk assessment standards. Environ Sci Eur 25:33 BioMed Central Full Text
Chronic Toxicity Studies. OECD Publishing, Paris.
chemicals. Int J Biol Sci 5:438-443 Publisher Full Text
in support of the safety of a genetically modified corn variety (MON 863). Food Chem Toxicol 45:2073-2085 Publisher Full Text
responses. Endocr Rev 33:378-455 Publisher Full Text
on health risks or nutritional assessment studies of genetically modified products. Food Policy 2011(36):197-203 Publisher Full Text
[http:/ / www.bfr.bund.de/ en/ the_bfr_has_finalised_its_draft_rep ort_for_the_re_evaluation_of_glypho sate-188632.html] webcite

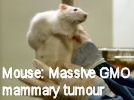
 French Prof Gilles-Eric Séralini's scientific study that identified grotesque tumour and other disease processes including severe liver and kidney damage in rats fed on 'Roundup ready' GMO maize has been republished in
French Prof Gilles-Eric Séralini's scientific study that identified grotesque tumour and other disease processes including severe liver and kidney damage in rats fed on 'Roundup ready' GMO maize has been republished in 
 The republication of the Séralini study is a triumph for science over the crushing trends of corporations to influence and silence significant scientific research. It is not surprising that it took a European study to expose these problems, since the Anglophone scientific press is so affected by upstream commercial selection of scientific research. The public hear so much about scientific studies but hardly appreciate that corporations are influencing what is studied, who leads teams and what gets funded and ultimately published in Australian and other universities. At back of this is the complicit mass media, which simply fails to promote information in the public interest if it contradicts commercial interests. If the Fairfax or Murdoch Press even attend to this crucial matter, we can be sure that the reporting will be short-lived. I would love to be proved wrong on this.
The republication of the Séralini study is a triumph for science over the crushing trends of corporations to influence and silence significant scientific research. It is not surprising that it took a European study to expose these problems, since the Anglophone scientific press is so affected by upstream commercial selection of scientific research. The public hear so much about scientific studies but hardly appreciate that corporations are influencing what is studied, who leads teams and what gets funded and ultimately published in Australian and other universities. At back of this is the complicit mass media, which simply fails to promote information in the public interest if it contradicts commercial interests. If the Fairfax or Murdoch Press even attend to this crucial matter, we can be sure that the reporting will be short-lived. I would love to be proved wrong on this.
Comments
Liz Rose (not verified)
Sat, 2014-07-12 12:55
Permalink
GMO pet food and tumours in cats and dogs
Anonymous (not verified)
Sun, 2014-08-10 20:02
Permalink
Glyphosphate birth defects - Farmer alarm re Monsanto Roundup
Add comment